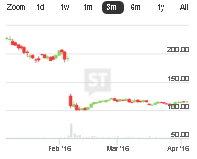
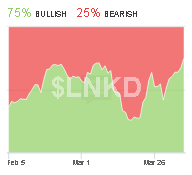
Our Sponsors
Our Sponsors
Sun Pharma - Live Stock Updates
livemint
@livemint
July 3rd, 2023 08:10
#Nifty, #Sensex surged to an all-time high this week on a closing basis after 7 months. https://t.co/k0bVsrNIeq
#Nifty, #Sensex surged to an all-time high this week on a closing basis after 7 months. https://t.co/k0bVsrNIeq
livemint
@livemint
July 3rd, 2023 08:10
#Nifty, #Sensex surged to an all-time high this week on a closing basis after 7 months. https://t.co/k0bVsrNIeq
July 3rd, 2023 08:10
#Nifty, #Sensex surged to an all-time high this week on a closing basis after 7 months. https://t.co/k0bVsrNIeq
I_am_Sayar
@I_am_Sayar
July 3rd, 2023 06:43
As Nifty climbs 19,100-mark, will pharma lead the way ahead? - [ Read here : https://t.co/RWhAe0xrZw ] #nse #bse… https://t.co/vDsb3e6MuI
As Nifty climbs 19,100-mark, will pharma lead the way ahead? - [ Read here : https://t.co/RWhAe0xrZw ] #nse #bse… https://t.co/vDsb3e6MuI
I_am_Sayar
@I_am_Sayar
July 3rd, 2023 06:43
As Nifty climbs 19,100-mark, will pharma lead the way ahead? - [ Read here : https://t.co/RWhAe0xrZw ] #nse #bse… https://t.co/vDsb3e6MuI
July 3rd, 2023 06:43
As Nifty climbs 19,100-mark, will pharma lead the way ahead? - [ Read here : https://t.co/RWhAe0xrZw ] #nse #bse… https://t.co/vDsb3e6MuI
SheelSourav68
@SheelSourav68
July 2nd, 2023 09:07
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
SheelSourav68
@SheelSourav68
July 2nd, 2023 09:07
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 2nd, 2023 09:07
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
vidit127
@vidit127
July 1st, 2023 16:55
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
vidit127
@vidit127
July 1st, 2023 16:55
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 1st, 2023 16:55
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
CRKOLTE
@CRKOLTE
July 1st, 2023 16:19
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
CRKOLTE
@CRKOLTE
July 1st, 2023 16:19
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 1st, 2023 16:19
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
ARUNKUM45728043
@ARUNKUM45728043
July 1st, 2023 16:02
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
ARUNKUM45728043
@ARUNKUM45728043
July 1st, 2023 16:02
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 1st, 2023 16:02
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
Pavan84059219
@Pavan84059219
July 1st, 2023 15:26
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
Pavan84059219
@Pavan84059219
July 1st, 2023 15:26
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 1st, 2023 15:26
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
BHAWANI33099915
@BHAWANI33099915
July 1st, 2023 15:08
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
BHAWANI33099915
@BHAWANI33099915
July 1st, 2023 15:08
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 1st, 2023 15:08
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
jogananda_nayak
@jogananda_nayak
July 1st, 2023 15:04
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
jogananda_nayak
@jogananda_nayak
July 1st, 2023 15:04
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…
July 1st, 2023 15:04
RT @ZeeBusiness: #StockMarket || इन 5 स्टॉक्स में होगी पैसों की बारिश! #StocksToBuy #stockstowatch #StockMarketindia #market #weekend Kn…